The affiliated team of CDH specialists offers patients and families the opportunity to participate in clinical trials, should they be interested. The team conducts many different types of trials and research, ranging from simple reviews of medical records and outcomes to complex therapy or intervention trials. Here are some examples of the research the team is involved in:
- Basic science research on the blood vessels of the lungs.
- Translational research introducing new therapies to patients.
- Clinical research doing chart reviews and following patient results.
- Multicenter collaborative research – our center coordinates the International CDH Study group, a consortium of centers who collaborate to evaluate outcomes and improve care.
Completed Clinical Trials
The affiliated team has participated in the following CDH-related clinical trials, which have been completed and the results have been published:
- TOTAL Trial* – Published in the New England Journal of Medicine
*The Fetal Center will continue offering the FETO intervention procedure to patients diagnosed with a severe CDH who qualify.
Research Trials
The team is conducting or participating in active, ongoing CDH trials:
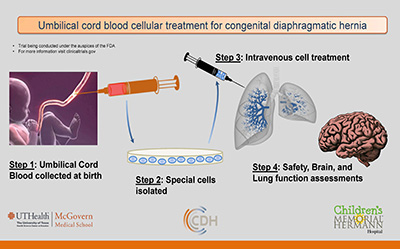
Umbilical Cord Blood Trial – offered to qualifying patients at time of delivery at Children’s Memorial Hermann Hospital
- Goal:Determine if special cells (mononuclear cells) from a child’s own UCB are safe and improve outcome
- Intervention: Autologous UCB given intravenously
- Timing: Cells isolated at birth & administered in the 1st week of life
- Follow-up: 2 years
Milrinone Trial – offered to qualifying patients in the Level IV Neonatal Intensive Care Unit (NICU) at Children’s Memorial Hermann Hospital
- Goal: Determine if a drug helps improve blood oxygenation
- Intervention: Administer a drug vs placebo intravenously
- Timing: Within the first week of life
- Follow-up: 1 year
iOS / Asthma Trial – offered to patients in the CDH Long-term Follow-up Clinic
- Goal: Determine if older children have specific restrictive or obstructive airway issues
- Intervention: None
- Timing: 2 years old – 18 years old
- Follow-up: as long as the child is in the clinic
The Fetal Center participates in ongoing research trials to advance medicine in the treatment of CDH and other conditions, with the goal of improving patient outcomes. The Fetal Center is one of only three U.S. centers to hold membership in all three key maternal-fetal research networks:
- National Institute of Child Health and Human Development (NICHD) Neonatal Research Network
- NICHD’s Maternal-Fetal Medicine Units (MFMU) Network
- North American Fetal Therapy Network (NAFTNet)
Pediatric surgeons in the division of General and Thoracic Pediatric Surgery at McGovern Medical School are internationally recognized for providing state-of-the-art neonatal critical care and minimally invasive surgical repair of CDH.
Congenital Diaphragmatic Hernia International Study Group and Registry
The care provided is based on data collected and new insights gained through the International Congenital Diaphragmatic Hernia Study Group and the CDH Registry, which reside at the hospital and medical school. In 1995, the CDH Study Group was founded by McGovern Medical School physicians, affiliated with Children’s Memorial Hermann Hospital, and the voluntary collaborative has gathered data on more than 13,000 babies with diaphragmatic hernia.
Overall worldwide survival of infants diagnosed with congenital diaphragmatic hernia (CDH) has increased from about 55% to above 70% in the last 30 years. Unfortunately, CDH remains a challenge for physicians, surgeons and scientists. Although the defect can be corrected with surgery, the arrest in prenatal development of the lungs results in unacceptably high rates of neonatal mortality and long-term complications for children born with CDH.
Thanks to more accurate prenatal diagnosis and the work of the international CDH Study Group – led by physicians affiliated with Children’s Memorial Hermann Hospital and McGovern Medical School – the global survival rate of infants born with CDH is steadily rising. Coordinated by Kevin P. Lally, MD, MS, and members from multiple specialties and centers, the CDH Study Group is a coalition of centers from around the world that track CDH patient outcomes in an international database – the CDH Registry. Based on risk-adjusted data, Children’s Memorial Hermann Hospital’s outcomes are in the top 10% or higher worldwide.
In the past 25 years since the CDH Study Group was founded, the voluntary collaborative has gathered data on more than 13,000 babies with CDH. The registry now represents centers in 16 countries. Information from this registry has been used in more than 50 CDH Study Group reports. These projects evaluate diagnostic and prognostic variables such as preductal oxygen saturation, defect size/anomaly association and pulmonary hypertension.
Investigators at Children’s Memorial Hermann Hospital are also engaged in promising research using extracellular vesicles, small membrane particles released from mesenchymal stem cells, to help manage pulmonary hypertension and reduce the mortality rate of CDH. While their research remains in the early stages, their aim is to translate innovative therapies to the clinical setting as quickly as possible.
Contact Us
If you have any questions, use the online tool below to help us connect with you. To refer a patient or schedule an appointment, please contact our clinic using the information below.
- The Fetal Center
UT Professional Building
6410 Fannin, Suite 210
Houston, Texas 77030
(832) 325-7288
Toll free: (888) 818-4818
Fax: (713) 383-1464
Email: thefetalcenter@memorialhermann.org - Pediatric General & Thoracic Surgery
6410 Fannin Street, Suite 950
Houston, Texas 77030
(832) 325-7234
Email: cdh@uth.tmc.edu
Office Hours: 8 a.m. to 5 p.m. (Monday-Friday except major holidays)
To contact Children's Memorial Hermann Hospital, please fill out the form below.